CNN
—
Small clinical trials suggest that drugs like Ozempic could be used not only to treat diabetes and weight loss, but also to protect the brain and slow the decline in thinking, memory, and daily activities in people with Alzheimer’s disease. Results must be supported by larger clinical trials already underway before the drug can be approved for use in Alzheimer’s.
The British study of 204 people with Alzheimer’s disease found that people who took the diabetes drug liraglutide (an earlier version of Ozempic’s class of drugs known as a GLP-1 receptor agonist) experienced 18% slower cognitive decline over a year than those who took a placebo.
But the trial did not achieve its main goal of changing the brain’s rate of glucose metabolism, and researchers suggested that this may be because of its small size. The results were published Tuesday. Alzheimer’s Association International Conference The study, conducted in Philadelphia, has not yet been published in a peer-reviewed journal.
“We’ve known for some time through animal studies that GLP-1 has different types of activity in the brain,” he says. Maria Carrillo“This study really proves that the potential is there,” said the Alzheimer’s Association’s chief scientific and medical officer, who was not involved in the study.
In addition to the cognitive benefits, the study also found that the drug reduced volume loss in several areas of the brain by 50 percent, according to a news release from the Alzheimer’s Association. Larger trials Carrillo told CNN that the project, run by Novo Nordisk, the manufacturer of Ozempic, will be successful.
Beyond Diabetes and Weight Loss
GLP-1 drugs exploded In recent years, it has been used for diabetes and weight loss, and has been shown to be beneficial for a variety of other health conditions, including protecting the heart and blood vessels. kidneyreduction Sleep apnea And potentially Poisoning.
the study Animal studies suggest that in the brain, the drug may reduce neuroinflammation, suppress toxic proteins called amyloid and tau, improve insulin resistance and enhance synaptic function – the transmission of impulses between cells, he said. Dr. Paul EdisonProfessor of Neuroscience at Imperial College London, who led the trial.
“This is the first study to really look at a relatively large number of patients to see if there’s any neuroprotective effect in Alzheimer’s disease,” Edison said.
The study involved participants who were primarily suffering from mild Alzheimer’s disease, which was measured with a test called the Mini-Mental State Examination. scale People with a score of 21 to 26 are considered to have mild Alzheimer’s, and the majority of participants in the new study scored around 22, but some fell as low as 17, indicating moderate Alzheimer’s, Edison said.
Diabetic patients were excluded because diabetes itself Risk Factors For Alzheimer’s disease.
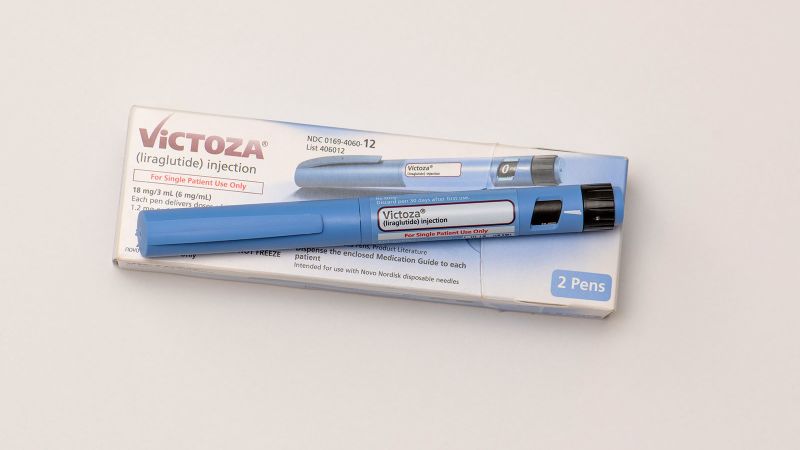
Edison and his team conducted the trial using liraglutide, a daily injection sold under the brand name. Victoza Diabetes and Saxenda According to him, the drug is said to be effective in weight loss because it resembles the GLP-1 hormone found in the human body, and when research on it began about 10 years ago, it was sold commercially as a diabetes treatment.
Ozempic uses the active ingredient semaglutide. approved It was introduced in the US in 2017 as a diabetes treatment, and later in the UK as a weight loss treatment. WegoviIt was approved in the United States in 2021. It is given as a once-weekly injection.
The GLP-1 drug class also includes Eli Lilly’s Maunjaro and Zepbound, which use the active ingredient tirzepatide, which mimics not only GLP-1 but another hormone called GIP, and a snowballing number of other companies are working on them. Even more powerful Medicine. Already existing medicines Shortage Businesses are trying to keep up with growing demand.
Lilly already has operations in the Alzheimer’s field. Get Approval The company this month announced a treatment called Xanra that removes amyloid plaque buildup in the brain, but has not announced any clinical trials of a GLP-1 drug for Alzheimer’s disease.
Lilly told CNN that it continues to evaluate future development options for tirzepatide, but has not announced any development plans for Alzheimer’s disease at this time.
Novo Nordisk, which sells liraglutide and semaglutide, is positioning the Alzheimer’s trial as a longshot bet, said CEO Lars Fruagaard Jorgensen. He told CNN “Alzheimer’s is one of the most difficult diseases to study and there have been many failed drug developments,” he said last year.
“So I just want to warn you,” he continued, “that this is probably the highest-stakes trial we’ve ever conducted.”
Novo Nordisk is conducting trials of a daily tablet version of semaglutide, with results expected in autumn 2025. An oral form is already sold under the brand name “semaglutide” for the treatment of diabetes. Rubelsus.
The Danish pharmaceutical giant, which published the study in 2020, Said The study was planned to enroll approximately 3,700 people with early-stage Alzheimer’s disease, with the primary treatment period lasting approximately two years.
The company later Investor Presentation The company said the decision to initiate Phase 3 trials was based on data including real-world evidence studies showing a lower risk of dementia in people taking GLP-1 drugs, analysis of effects seen in other clinical trials, and animal studies showing the drug is associated with improved memory function, reduced neuroinflammation and a systemic anti-inflammatory effect.
Get the CNN Health weekly newsletter
Earlier this month, review Researchers from Oxford University in the US looked at patient records and found that semaglutide was associated with a lower risk of cognitive impairment and nicotine addiction. The study was designed to assess whether the drug had any adverse effects on the brain, but actually found the opposite.
GLP-1 drugs include However, side effects are primarily gastrointestinal upset, including nausea and vomiting, which were the most common side effects observed in trials of liraglutide in Alzheimer’s disease.
The research was funded by Novo Nordisk, as well as Alzheimer’s Society UK and others.
More research is needed to prove that GLP-1 drugs are effective for Alzheimer’s patients, but Carrillo cautioned that the drugs may not only be used alone but also in combination with other medications. Recently Approved A drug that removes amyloid plaque buildup from the brain.
“Not only have the clinical trials of semaglutide been successful, but there is also great excitement about the possibility of combining it with monoclonal antibodies that are now FDA-approved,” she said.