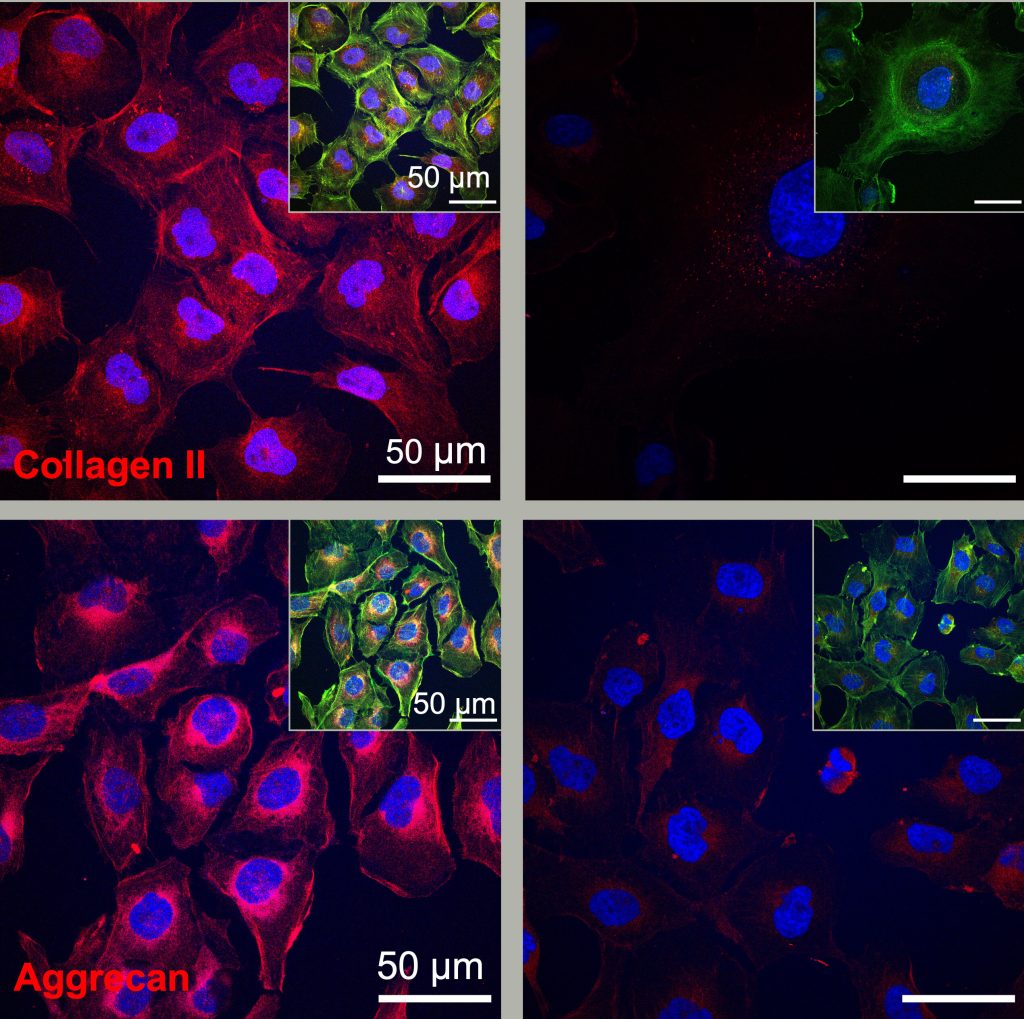
Chondrocytes produce more protein components (collagen II and aggrecan) for regeneration when treated with fast-moving molecules (left) compared to slower-moving molecules. Credit: Stupp Research Group/Northwestern University
In November 2021, researchers at Northwestern University introduced a new injectable treatment that uses fast-moving “dancing molecules” to repair tissue and reverse paralysis. Severe spinal cord injury.
The same research group is now applying this therapeutic strategy to injured humans. cartilage In the new study, the treatment activated gene expression required for cartilage regeneration within just four hours, and after just three days, human cells were producing the protein components required for cartilage regeneration.
The researchers also Molecular Motion The molecular “dance” movement was key to inducing the cartilage growth process.
of The study was published today In Journal of the American Chemical Society
“When we first observed the therapeutic effects of dancing molecules, we saw no reason why they should apply only to the spinal cord,” said study leader Samuel I. Stapp of Northwestern University. “Now we’ve seen them in two types of cells that are completely separate from each other: cartilage cells in the joints and neurons in the brain and spinal cord. This strengthens our belief that we may have discovered a universal phenomenon that could be applicable to many other tissues.”
Stapp, an expert in regenerative nanomedicine, is a Board Chair Professor of Materials Science and Engineering, Chemistry, Medicine and Biomedical Engineering at Northwestern University and founding director of the university’s Simpson-Querrey Institute for Bionanotechnology and its affiliated center, the Center for Regenerative Nanomedicine. Stapp is affiliated with the McCormick School of Engineering, the Weinberg School of Letters and Science, and the Feinberg School of Medicine. Shelby Yuan, a graduate student in the Stapp lab, is the lead author of the study.
Big problems, few solutions
As of 2019, Approximately 530 million people worldwide According to the World Health Organization, approximately one million people worldwide suffer from osteoarthritis, a degenerative disease in which joint tissue is destroyed over time, making it a common health problem and a leading cause of disability.
For people with severe osteoarthritis, the cartilage can become so thin that the joints become bone-on-bone contact with no cushioning between the bones. Not only is this extremely painful, but the patient’s joints no longer function properly. The only effective treatment is then joint replacement surgery, which is expensive and highly invasive.
“Current treatments aim to slow the progression of the disease or postpone inevitable joint replacement,” Stapp says, “but because humans do not have the natural ability to regenerate cartilage once they reach adulthood, regeneration is not an option.”
What are “dancing molecules”?
Stapp and his team hypothesized that “dancing molecules” could stimulate the regeneration of stubborn tissue. Dancing molecules, previously invented in Stapp’s lab, are assemblies that form synthetic nanofibers of tens to hundreds of thousands of molecules that send powerful signals to cells. By coordinating their collective motion through their chemical structure, Stapp found that the moving molecules could rapidly find and properly engage their targets. Cell ReceptorsThese too are constantly moving and are very densely packed on the cell membrane.
Once inside the body, the nanofibers mimic the extracellular matrix of the surrounding tissue: by matching the structure of the matrix, mimicking the movement of biomolecules and incorporating bioactive signals into receptors, the synthetic material is able to communicate with cells.
“Cell receptors are constantly on the move,” Stapp says, “by moving molecules around, dancing, or even temporarily popping out of these structures.” Supramolecular PolymersIt allows you to connect with your receptors more effectively.”
Movement is important
In the new study, Stapp and his team focused on the receptor for a specific protein that is important in the formation and maintenance of cartilage. To target this receptor, the team developed a new cyclic peptide that mimics the bioactive signal of the protein, called transforming growth factor beta 1 (TGFb-1).
The researchers then incorporated this peptide into two different molecules that interact in water to form a supramolecular polymer, each with the same ability to mimic TGFb-1. The researchers engineered one supramolecular polymer with a special structure that allowed the molecule to move more freely within the large assembly, while the other supramolecular polymer restricted the molecule’s movement.
“We wanted to modify the structure to compare two systems with different degrees of motion,” Stapp says. “The strength of the supramolecular motion in one is much greater than that of the other.”
Both polymers mimicked the signals that activate the TGFb-1 receptor, but the polymers with the fast-moving molecules were much more effective — in some ways, they were more effective than the proteins that naturally activate the TGFb-1 receptor.
“After three days, human cells exposed to the longer aggregates of more mobile molecules produced more Protein Ingredients “For the production of collagen II, one of the essential components of cartilage regeneration, a dance molecule containing a cyclic peptide that activates the TGF-β1 receptor was more effective than the natural protein that has this function in biological systems,” Stapp said.
What’s next?
Stupp’s team is now testing these systems in animal studies and adding additional signals to develop advanced bioactive therapies.
“Given our successful work with human chondrocytes, we predict that its use in advanced translational preclinical models will significantly enhance cartilage regeneration,” Stapp said, “leading to a new bioactive material for the regeneration of cartilage tissue in joints.”
Stapp’s lab is also testing the dancing molecules for their bone regeneration potential, with already promising early results that will likely be published later this year. At the same time, he is testing the molecules in human organoids to speed up the process of discovering and optimizing therapeutic materials.
Stapp’s team is also pursuing applications from the Food and Drug Administration in hopes of gaining approval for clinical trials to test the spinal cord repair therapy.
“It appears that these fundamental discoveries about ‘dancing molecules’ can be applied to a wide range of conditions,” Stapp said. “Controlling supramolecular motion through chemical design appears to be a powerful tool to enhance the efficacy of a variety of regenerative therapies.”
For more information:
Shelby C. Yuan et al. “Supramolecular Motion Enables Chondrogenic Activity of a Cyclic Peptide Mimetic of Transforming Growth Factor-β1” Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c05170
Provided by
Northwestern University
Quote: New study shows ‘dancing molecules’ can regenerate cartilage in 3 days (July 26, 2024) Retrieved July 27, 2024 from https://medicalxpress.com/news/2024-07-molecules-regenerate-cartilage-days.html
This document is subject to copyright. It may not be reproduced without written permission, except for fair dealing for the purposes of personal study or research. The content is provided for informational purposes only.