A new study has identified brain neurons that distinguish between fullness and nausea in obesity drugs, paving the way for treatments that suppress appetite without causing side effects.
Research suggests that new drugs may be developed that suppress appetite without causing nausea.
The next chapter for the popular, buzzworthy obesity drug may focus on understanding post-meal satiety and the brain’s regulation of nausea. Monell Chemical Senses Center They identified populations of neurons in the brain that control food intake without causing nausea in animal models and distinguished between the beneficial and side effects of these drugs.
The study was published in the journal Naturerepresent two distinct neural circuits that control different effects of the same drug. The drug studied is one of the most effective weight loss drugs currently available, known as a long-acting glucagon-like peptide 1 receptor (GLP1R) agonist, which initiates a neurochemical response via receptors expressed in the body.
One of the most effective and popular GLP1-based drugs, semaglutide, marketed as Ozempic and Wegovy, has produced impressive weight loss results in clinical trials. According to the World Health Organization, one in eight people worldwide will be obese by 2022, making the development of such drugs crucial.
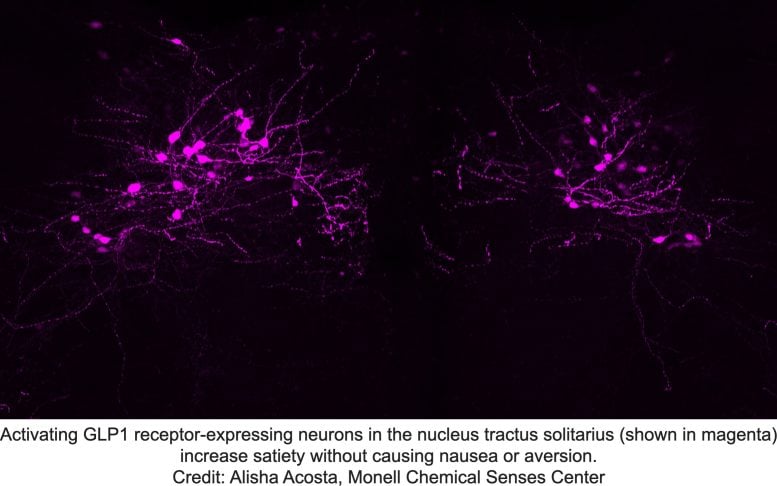
Activating GLP1 receptor-expressing neurons in the nucleus of the solitary tract (magenta) increases satiety without causing nausea or disgust. Credit: Alisha Acosta, Monell Chemical Senses Center
Overcoming side effects in obesity treatment
“One of the barriers to drug treatments for obesity are side effects such as nausea and vomiting,” said Monell assistant professor and senior author Amber L. Alhadeff, PhD. “We didn’t really know whether these unpleasant side effects were related to or even necessary for the weight loss benefits.”
To find the answer, the Monell team investigated the brain circuits that connect the feeling of fullness after consuming a meal with the brain circuits that cause food avoidance due to nausea. The researchers found that neurons in the hindbrain mediate the effects of both of these obesity drugs and, unexpectedly, found that the individual neurons that mediate fullness and nausea are distinct.
two-photon Imaging of hindbrain GLP1R neurons in live mice revealed that most individual neurons are tuned to respond to either nutrient or aversive stimuli, but not both. Rear Area are more responsive to aversive stimuli, whereas GLP1R neurons in another area Nucleus of the solitary tract Lean into nutritional stimulation.
The team then manipulated the two GLP1R neuron groups separately to understand their impact on behavior. They found that activating neurons in the nucleus of the solitary tract induced satiety without aversive behavior, whereas activating neurons in the area postrema induced a strong aversive response. Importantly, obesity drugs reduced food intake even when the aversive pathway was inhibited. This surprising finding highlights the neuronal population in the nucleus of the solitary tract as a target for future obesity drugs that reduce food intake without making people feel sick.
“Developing experimental obesity drugs that selectively activate this population may promote weight loss while avoiding aversive side effects,” Alhadeff said. Indeed, the concept of separating therapeutic and adverse effects at the neural circuit level could theoretically be applied to any drug with side effects, the authors say.
Reference: “Separable hindbrain GLP1R circuits for satiety and aversion” by Kuei-Pin Huang, Alisha A. Acosta, Misgana Y. Ghidewon, Aaron D. McKnight, Milena S. Almeida, Nathaniel T. Nyema, Nicholas D. Hanchak, Nisha Patel, Yenoukoume SK Gbenou, Alice E. Adriaenssens, Kevin A. Bolding, and Amber L. Alhadeff, July 10, 2024; Nature.
Publication date: 10.1038/s41586-024-07685-6
This study National Institutes of Health (R00DK119574 and DP2AT011965), the American Heart Association, the New York Stem Cell Foundation, the Klingenstein and Simons Foundations, the Pew Charitable Trusts, the National Science Foundation (grant 2236662), the Pennsylvania Institute of Diabetes, Obesity and Metabolism, and the Monell Chemical Senses Center. The confocal microscope used in these studies was purchased with an NIH equipment grant (S10OD030354). Alhadeff is a Robertson Investigator at the New York Stem Cell Foundation and a Pew Biomedical Scholar.


