Federal health officials are warning that people are overdosing on certain products marketed for weight loss.
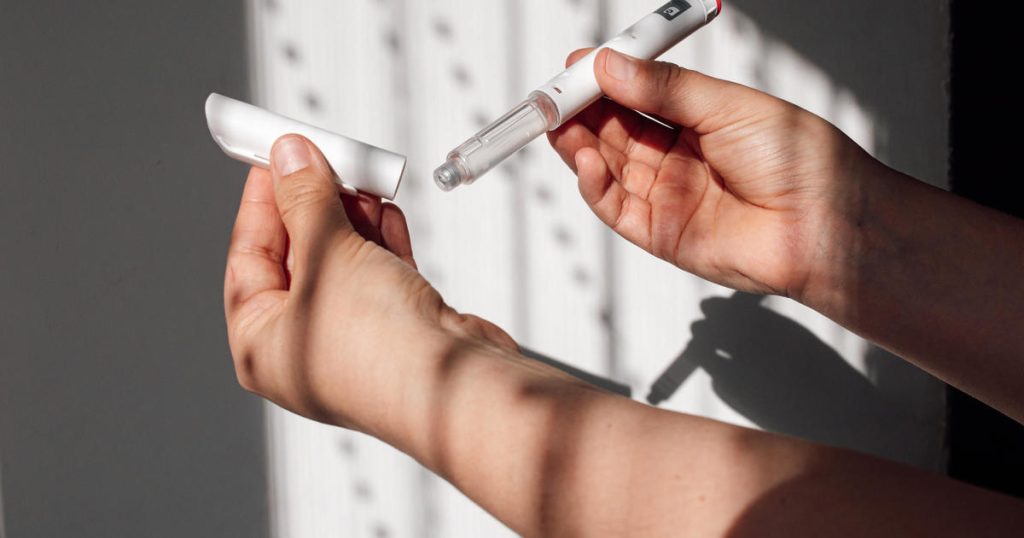
The FDA said it has received reports of patients needing medical attention or hospitalization due to administration errors with semaglutide injections dispensed in multi-dose vials.
The medication errors occurred as a result of people measuring out the wrong amount and self-administering the medication, or health care workers miscalculating the dosage of cheaper compounded versions of weight-loss drugs like Wegobee, the department said in a statement Friday. caveat.
According to the FDA, symptoms of overdose may include nausea, vomiting, abdominal pain, fainting, headache, migraine, dehydration, acute pancreatitis and gallstones.
The majority of reports involved patients accidentally drawing up more semaglutide than prescribed from multi-dose vials and self-administering 5 to 20 times the intended dose. Many reports involved patients being unfamiliar with how to use the syringe to measure out the intended dose.
U.S. Food and Drug Administration
The FDA said confusion over different units of measurement, such as milliliters and milligrams, may have contributed to the dosing error.
According to the FDA, one healthcare provider intended to administer 0.25 milligrams, or five units, but instead prescribed 25 units, resulting in patients receiving five times the intended amount and experiencing severe vomiting. Another healthcare provider prescribed 20 units instead of two units, resulting in three patients receiving 10 times the intended amount and experiencing nausea and vomiting.
There are currently three FDA-approved semaglutide products: Ozempic and Wegovy injections and Rybelsus tablets.
The agency said semaglutide combination products commonly sold by online pharmacies come in a variety of containers and packaging, including multi-dose vials and pre-filled syringes.
Compounded medicines High risk The agency said in its warning that the drugs may be more beneficial to patients than FDA-approved medications and should only be used when FDA-approved drugs cannot meet a patient’s medical needs.