The U.S. Food and Drug Administration has approved two injectable forms of the blockbuster weight loss and diabetes treatment drug semaglutide (Wegovy and Ozempic). Both come in prefilled pen form with preset doses, clear instructions for use, and information about overdose. But given the drug’s high cost and limited supply, many patients are turning to counterfeit versions that don’t always have the same safety precautions.
in Warning FridayThe FDA warned that overdoses were occurring with off-brand semaglutide injections that were dispensed in different strengths by compounding pharmacies, labeled with different units of measurement, administered in improperly sized syringes, and prescribed with incorrectly calculated doses.These errors have led some patients to receive as much as 20 times the intended amount of semaglutide, according to the FDA report.
The agency has not released the number of reported overdoses, but has received reports of people becoming ill after taking the medication incorrectly, some of whom have required hospitalization. An overdose of semaglutide can cause nausea, vomiting, abdominal pain, fainting, headaches, migraines, dehydration, acute pancreatitis and gallstones, the agency reported.
Something’s wrong with the calculation
In a typical situation, a compounding pharmacy will provide a customized prescription for an FDA-approved drug if a patient is allergic to a particular ingredient, needs a special dosage, or wants a liquid version of a drug instead of a tablet. But if there is a shortage of an over-the-counter drug (as there is currently for semaglutide), compounding pharmacies can legally step in and make their own versions, provided certain conditions are met. However, these knockoffs are not FDA-approved and therefore not the same thing. Assurance of safety, quality and efficacy as an approved medicine.
In its Friday warning, the FDA said some patients received confusing instructions from compounding pharmacies instructing them to inject a set number of semaglutide “units,” rather than milligrams or milliliters. The instructions stated the amount can vary depending on the concentration, but not milligrams or milliliters. In other instances, patients received a U-100 (1 milliliter) syringe to administer 0.05 milliliters of the drug, or five units. Due to the relatively large size of the syringe compared to the dosage, some patients administered 50 units instead of 5 units.
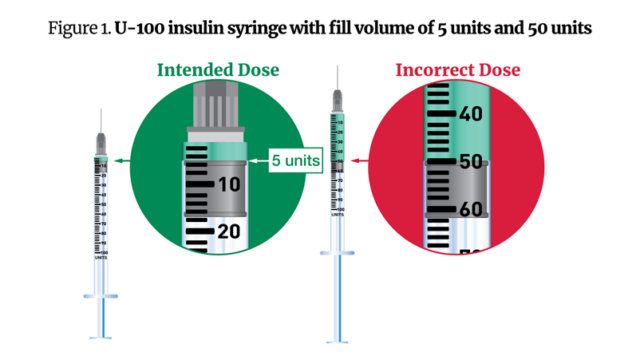
Meanwhile, FDA-approved semaglutide drugs are dosed in milligrams and come in standard concentrations. The FDA has received reports of health care professionals incorrectly converting milligrams to units or milliliters and calculating the wrong dosage. These calculation errors resulted in some patients receiving 5 to 10 times more semaglutide than intended.
“FDA recognizes that there is significant consumer interest in using combined semaglutide products for weight loss,” the agency wrote. “However, combined drugs pose higher risks to patients than the FDA-approved drug.” The agency urged patients and prescribers to use the combined version only when absolutely necessary.