Kobe University’s discovery of a new PGC-1⍺ protein variant that is more active during exercise and can regulate fat burning and energy metabolism suggests a breakthrough in treating obesity by increasing energy expenditure rather than simply reducing calorie intake.
New research findings highlight that PGC-1⍺ variants “b” and “c” are key to improving fat burning and energy metabolism during exercise, opening up new avenues for the treatment of obesity.
Some people lose weight more slowly than others after exercise, and a research team from Kobe University has discovered why. They studied what happens to mice that are unable to produce a signaling molecule that specifically responds to short-term exercise and regulates the body’s energy metabolism. These mice consume less oxygen and burn less fat during exercise, and are therefore more likely to gain weight. The research team also found this connection in humans, so new knowledge about this mechanism may provide a route to treating obesity.
The relationship between exercise and fat burning
It’s well-known that exercise burns fat. But some people find this much harder than others, leaving many in doubt whether the mechanism by which weight is lost or gained is as simple as “calories in minus calories out.” Researchers have previously identified a signaling molecule that seems to link exercise to its benefits – a protein named “PGC-1⍺.” But whether increasing amounts of this protein actually lead to these benefits remains inconclusive, as some experiments suggest this is the case while others don’t.
More recently, endocrinologist Wataru Ogawa of Kobe University and other researchers discovered that there are actually several different versions of this protein. “These new PGC-1α versions, called ‘b’ and ‘c’, have almost the same function as the conventional ‘a’ version, but are produced 10 times more in muscle during exercise, whereas the a version shows no such increase,” Ogawa explains. So his team set out to prove the idea that it is the newly discovered version, and not the previously known version, that regulates energy metabolism during exercise.
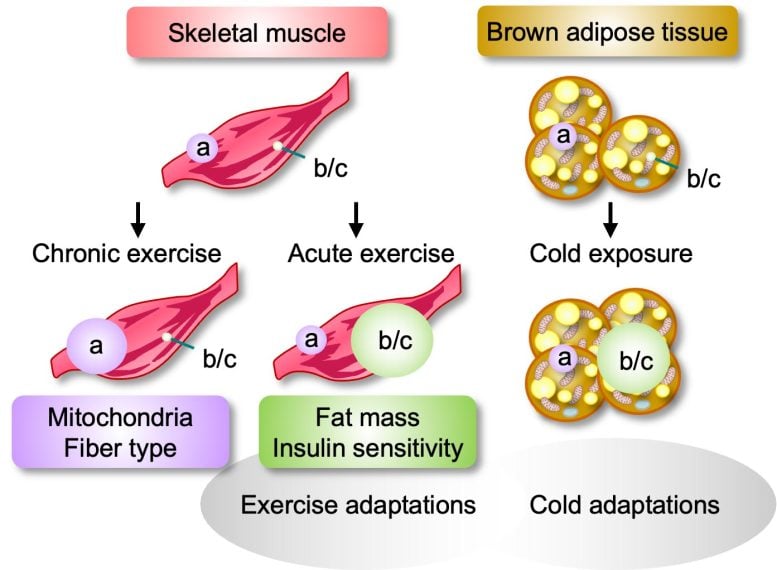
Different versions of the signaling molecule PGC-1⍺ respond to different stimuli: the canonical version (“a”) is produced in response to long-term exercise, and the alternative version (“b/c”) is produced in response to short-term exercise or cold exposure. A deficiency in these versions makes it harder for affected individuals to respond to these stimuli by burning fat or building muscle mass. Credit: K. Nomura et al. Published by Elsevier GmbH. DOI: 10.1016/j.molmet.2024.101968
To do so, the researchers created mice lacking the b and c versions of the signaling molecule PGC-1⍺ but retaining the standard a version, and then measured muscle growth, fat burning, and oxygen consumption in the mice at rest and during short- and long-term exercise. They also recruited human subjects, with or without type 2 diabetes, and subjected them to the same tests as the mice. InsulinIntolerant and obese people are known to have reduced levels of signaling molecules.
Biological meaning of protein variants
Ogawa and his team published their results in the journal Neurology. Molecular MetabolismThe researchers found that while all versions of the signaling molecule elicit similar biological responses, differences in their production levels have far-reaching effects on the health of the organism. The lack of alternative versions of PGC-1⍺, b and c, means that the organism is essentially blind to short-term activity and does not adapt to these stimuli, resulting in such individuals consuming less oxygen and burning less fat during and after exercise.
The team found that in humans, the more the subjects produced the b and c types of the signaling molecules, the higher their oxygen consumption and the lower their body fat percentage, both in healthy people and in patients with type 2 diabetes. “Therefore, the hypothesis that skeletal muscle genes determine obesity susceptibility was correct,” Ogawa summarises the findings.
But the researchers also found that chronic exercise stimulated the production of the canonical a version of PGC-1⍺, and that mice that exercised regularly for six weeks showed increased muscle mass, regardless of whether they were able to produce the alternative version of the signaling molecule.
Wataru Ogawa, an endocrinologist at Kobe University, has uncovered the physiological role of different versions of a signaling molecule that links exercise and its effects. They found that mice lacking a newly discovered version of the signaling molecule that is produced specifically in response to short-term exercise burned less fat than mice with both versions. Photo by Wataru Ogawa
Long-term effects and cold tolerance
The Kobe University research team looked at how production of different versions of PGC-1⍺ in fat tissue changed in addition to production in muscle and found no relevant effects on exercise. However, because animals also burn fat to maintain body temperature, the researchers also investigated the cold tolerance of mice.
Indeed, they found that exposure of animals to cold increases the production of the b and c forms of the signaling molecule in brown adipose tissue, and that the body temperature of individuals unable to produce these versions drops significantly under these conditions. On the one hand, this could contribute to the increase in body fat in these individuals, but on the other hand, it seems to suggest that the b and c forms of the signaling molecule may be involved in metabolic adaptation to short-term stimuli more generally.
Potential Treatment for Obesity
Ogawa and his colleagues point out that understanding the physiological activity of the different versions of PGC-1⍺ may lead to the development of treatments for obesity. “Recently, anti-obesity drugs that suppress appetite have been developed and are being prescribed in many countries around the world. However, there are no drugs that treat obesity by increasing energy expenditure. If we can find a substance that increases the b and c versions, this could lead to the development of drugs that increase energy expenditure during or without exercise. Such drugs may be able to treat obesity independently of dietary restrictions.”
The team is currently conducting studies to further investigate the mechanism by which production of the b and c versions of the signaling molecule increases during exercise.
Reference: “Adaptive gene expression of alternatively spliced variants of PGC-1α controls whole-body energy metabolism” Kazuhiro Nomura, Shinichi Kinoshita, Nao Mizusaki, Yoko Senga, Tsutomu Sasaki, Tadahiro Kitamura, Hiroshi Sakagami, Aki Emi, Tetsuya Hosooka, Masahiro Matsuo, Hitoshi Okamura, Taku Amano, Alexander M. Wolf, Naomi Uemura, Shigeo Ota, Tomoo Ito, Yoshitake Hayashi, Hiroshi Kiyonari, Anna Crook, Juleen R. Zealace, Wataru Ogawa, June 15, 2024, Molecular Metabolism.
DOI: 10.1016/j.molmet.2024.101968
This research was funded by the Japan Society for the Promotion of Science (grants 26461337, 16H01391, and 15H04848) and was carried out in collaboration with researchers from Tokushima University, Karolinska Institutet, Kyoto University, Gunma University, National Defense Academy of Japan, Nippon Medical School, RIKEN Center for Biosystems Dynamics Research, and the Asahi Life Foundation.


